On May 25, 2023, the Suzhou branch of Jiangsu Medical Device Inspection Institute's Usability Testing and Research Center completed the installation of the Cardiovascular Interventional Device Usability Testing System, marking a new milestone in the usability testing of cardiovascular interventional devices in China. As the first medical device usability testing platform in China, the Jiangsu Medical Device Usability Testing Platform will provide strong support for the usability research and testing of cardiovascular interventional devices in China.
The cardiovascular interventional device usability testing system was developed by the Jiangsu Medical Device Usability Testing Research Center and designed and manufactured by the professional surgical simulator manufacturer Preclinic Medtech (Shanghai) Co., Ltd. It can meet the requirements of human-computer interaction, ergonomics, ergonomics, usability testing, and user experience testing of cardiovascular interventional devices. This innovative testing system has completed the application for invention patents.
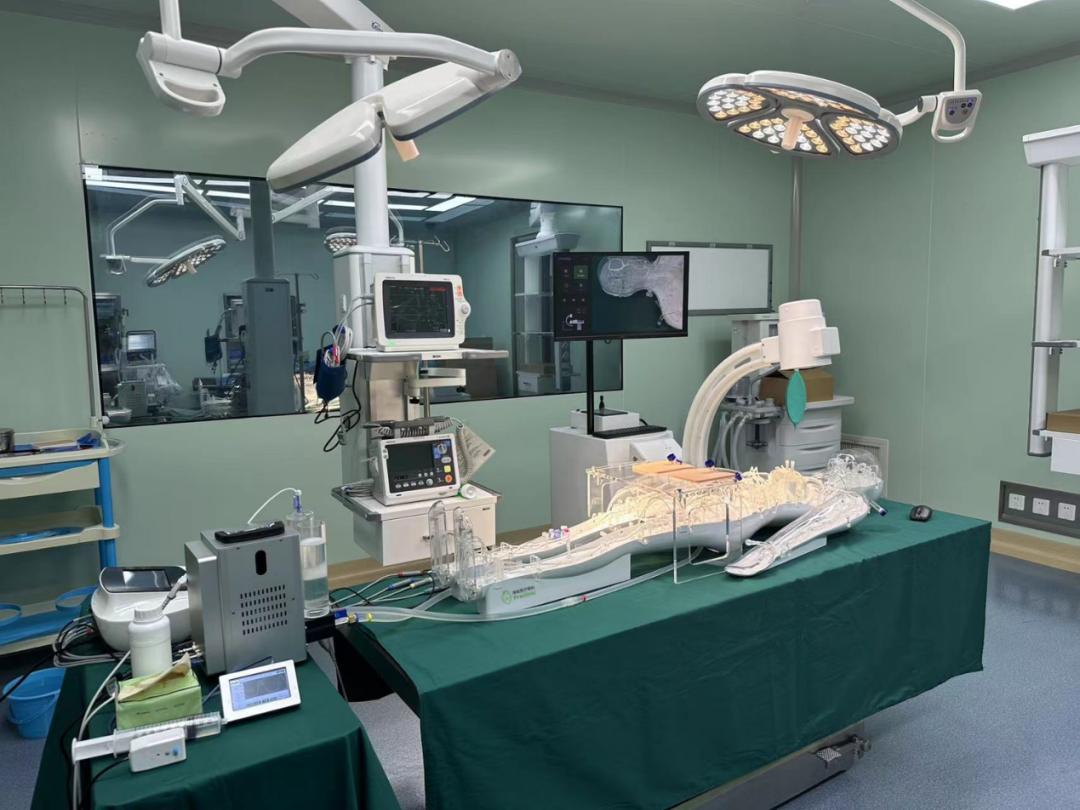
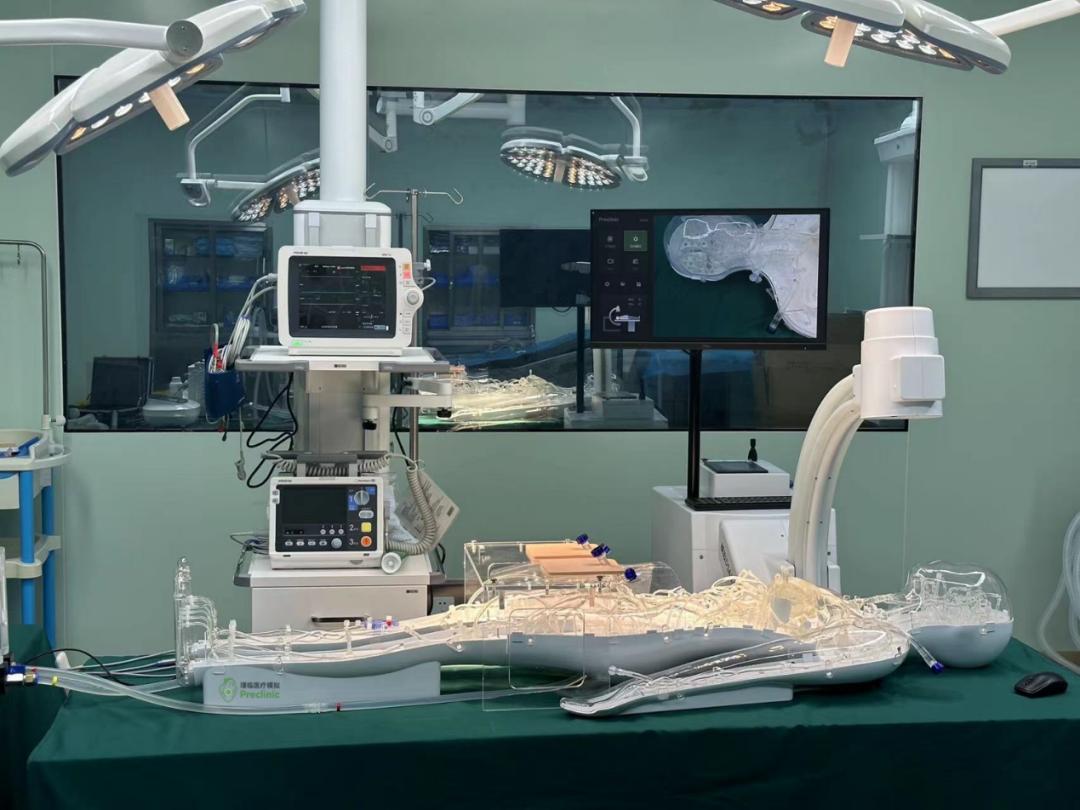
Medical device usability testing involves representative users using devices to perform representative tasks in order to reveal the device's interaction advantages and improvement opportunities. Its purpose is to understand whether the device meets user requirements and, most importantly, whether it can meet the requirements for safe operation through stress testing or troubleshooting of the device's user interface. By making design changes, the aim is to improve and confirm the design, ensuring that it does not lead to dangerous use errors. Usability testing can identify opportunities for improvements that make medical device operation simpler, safer, more effective, and user-friendly. This improvement in human-computer interaction quality benefits everyone associated with a given device. The most profound reason for conducting medical device usability testing is to prevent harm or death from user errors. The results of usability testing serve as the basis for design and development changes and are documented.

The Jiangsu Medical Device Usability Testing Platform is the first shared platform jointly built by the government, enterprises, universities, and international certification bodies in China. It is dedicated to improving the design level of medical devices and reducing the risks associated with their use. It plays an important role in enhancing the technological support level of regulatory authorities and promoting the high-quality development of the medical device industry in Jiangsu and even the whole country. It is an effective attempt to integrate China's innovation resources and explore the construction of shared platforms.
Currently, the platform has become China's first professional fully simulated medical environment usability testing platform. It can conduct various tests related to interaction design, human-computer interaction, human-computer interfaces, ergonomics, ergonomics, usability testing, and user experience. It achieves a user-centered approach and possesses the professional standards of medical device usability testing, making it a globally leading laboratory.


