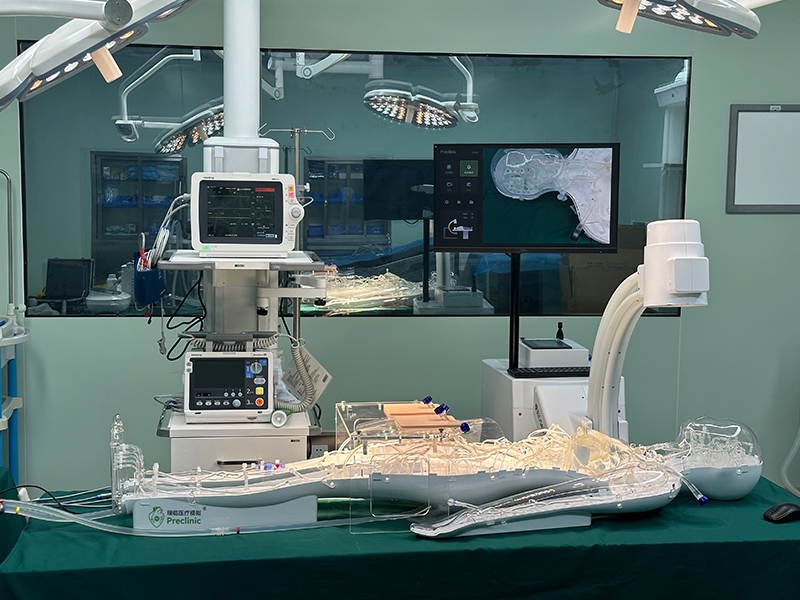
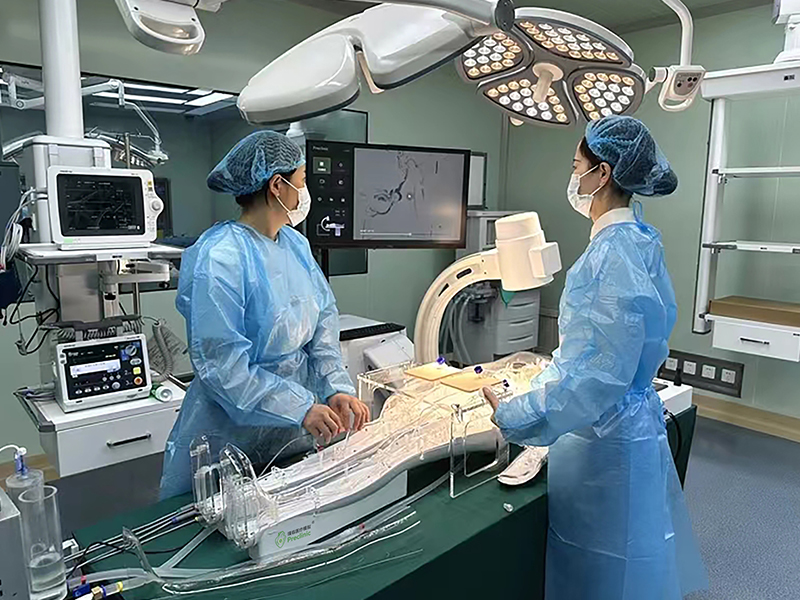
The first domestic usability testing system for cardiovascular interventional devices in China, developed by the Suzhou Laboratory Usability Testing Research Center of Jiangsu Medical Device Inspection Institute, has been successfully completed and put into trial use, marking a new level of usability testing for cardiovascular interventional devices in China. As the first medical device usability testing platform in China, the Jiangsu Medical Device Usability Testing Platform will provide strong support for the usability research and testing of cardiovascular interventional devices in China.
This cardiovascular intervention device usability testing system can meet multiple testing requirements for cardiovascular implant intervention devices, including human-computer interaction, human factors engineering, ergonomics, usability testing, and user experience. The innovative testing system has completed the patent application for invention.